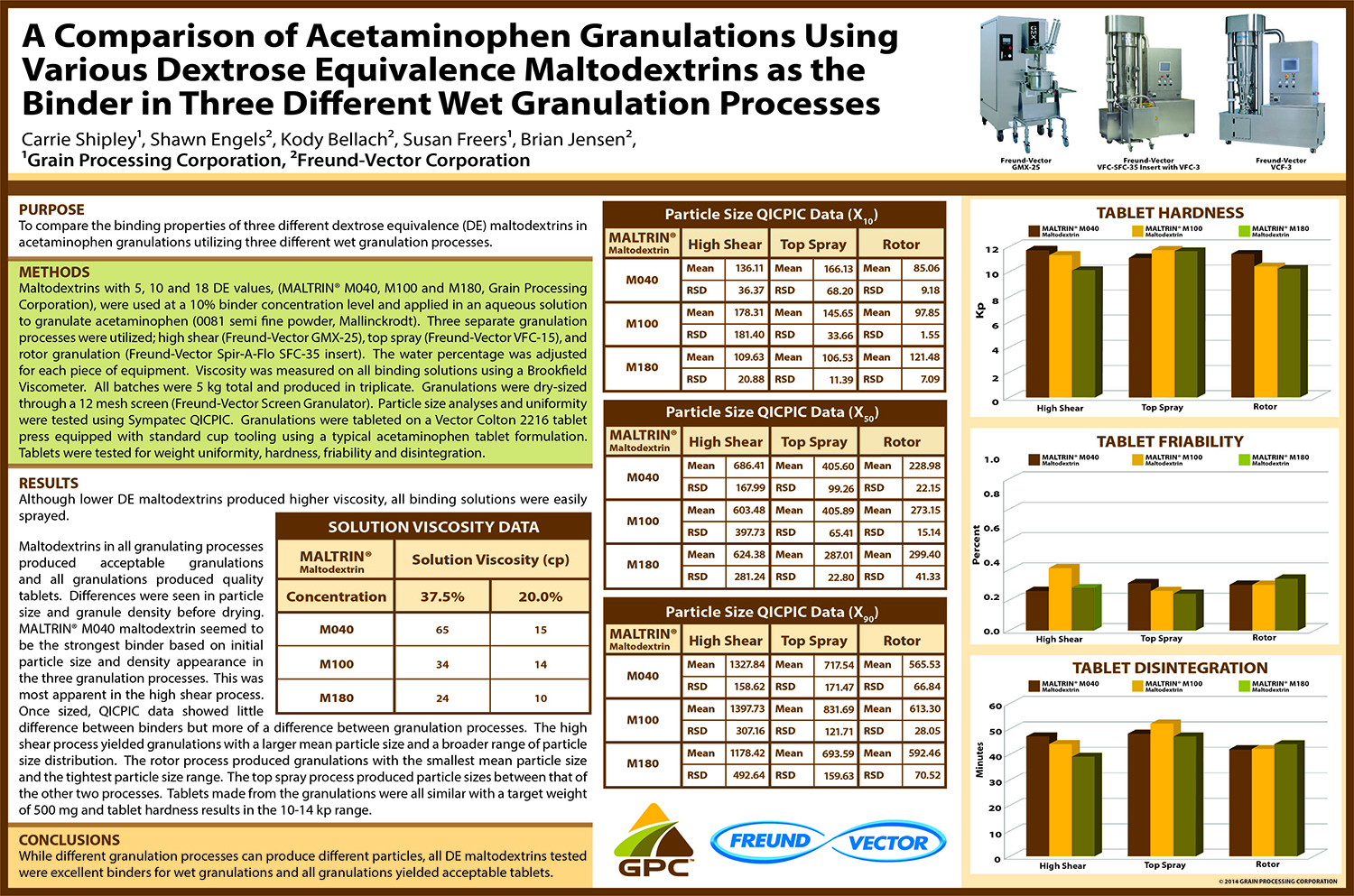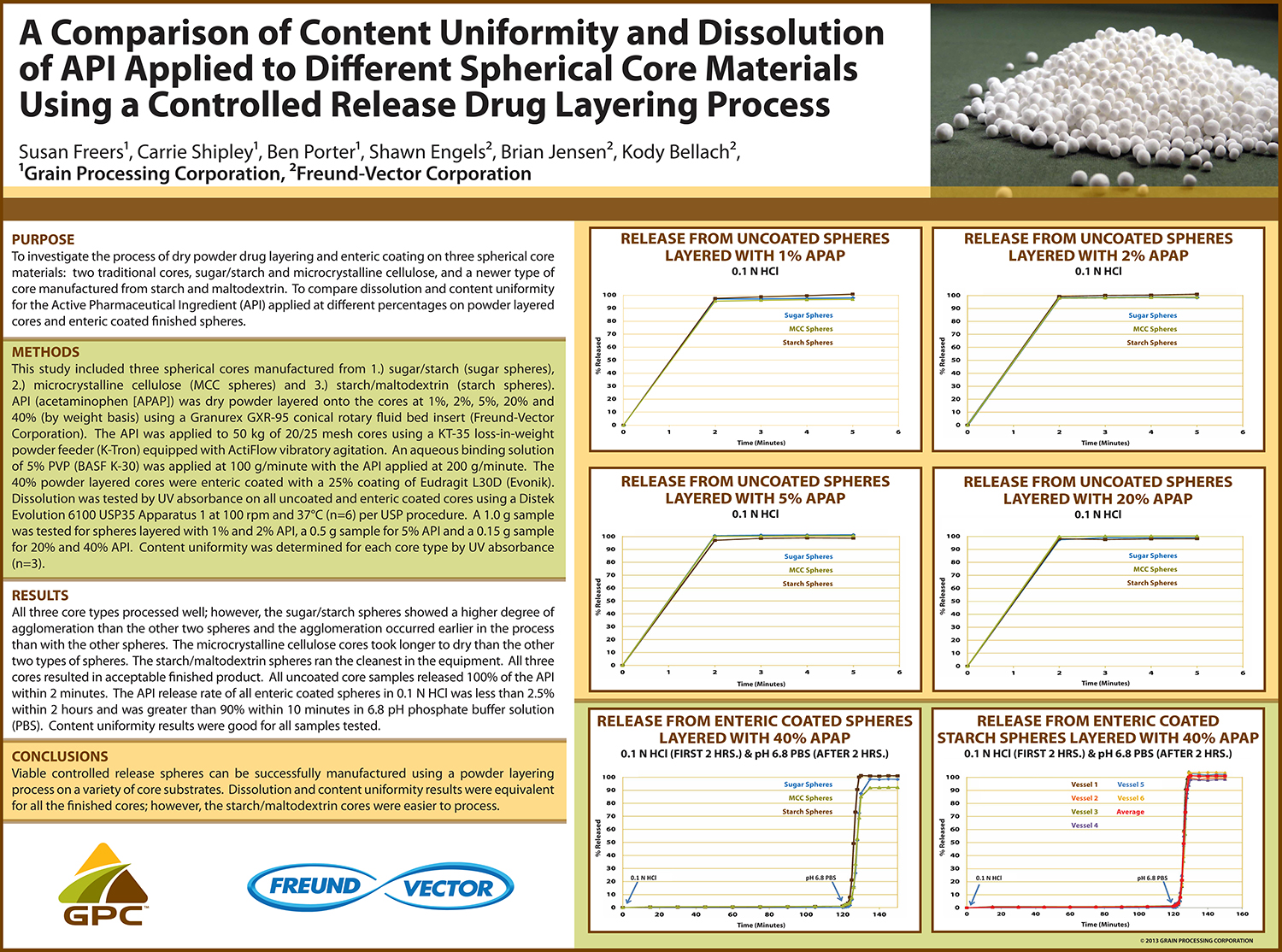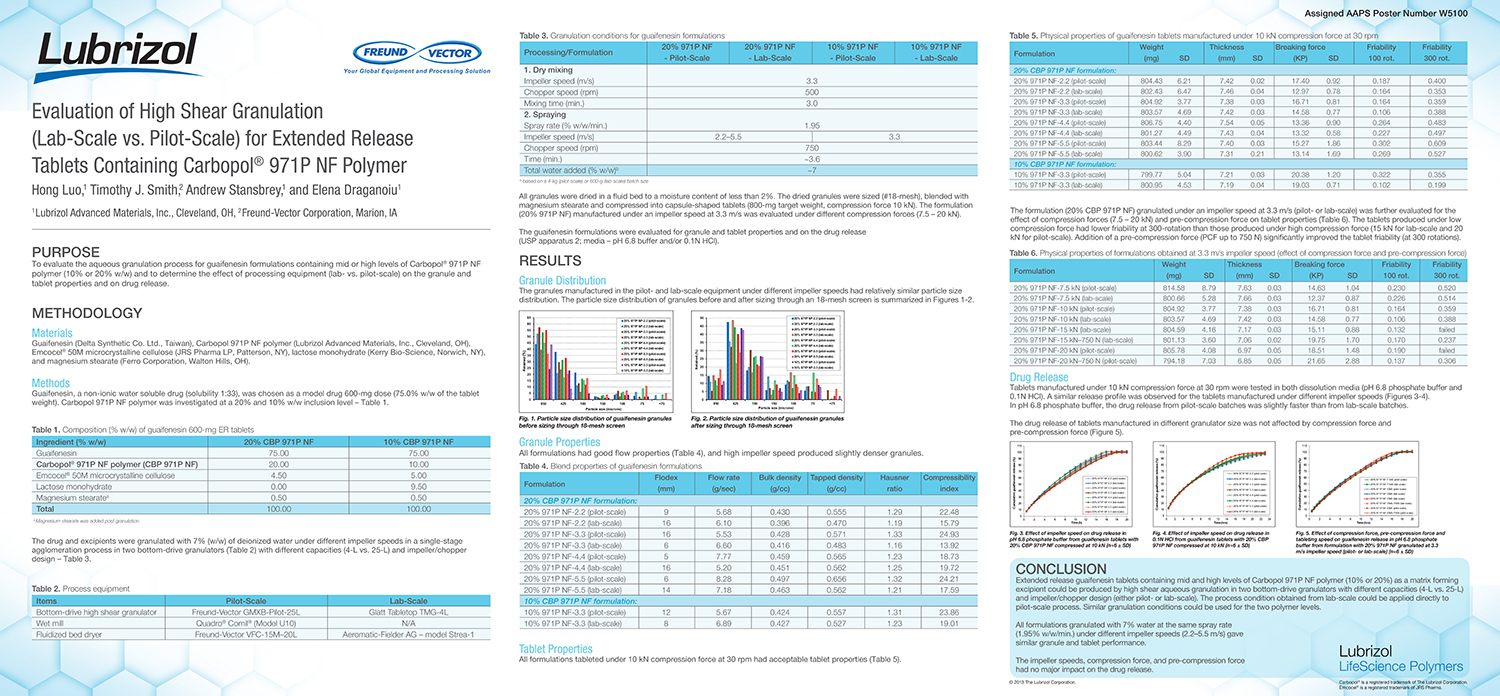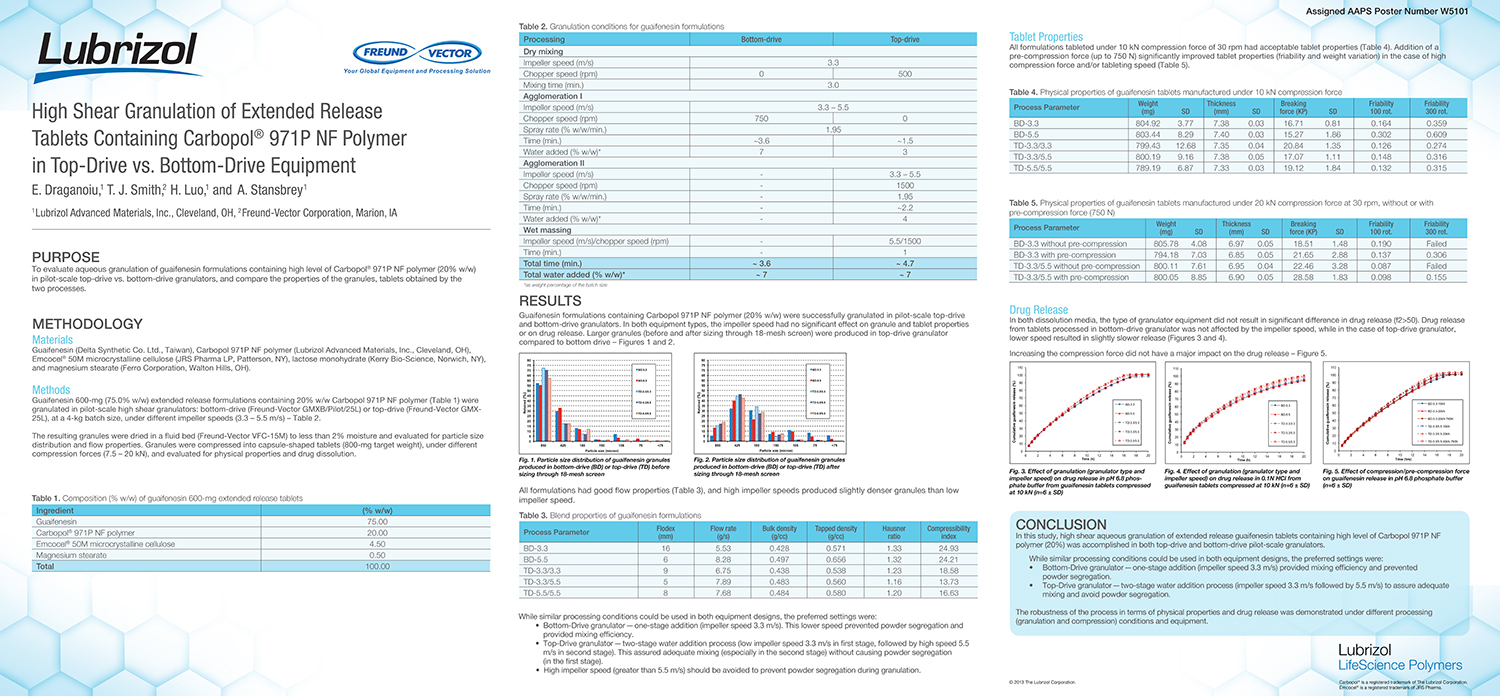
Utilization of Starch Micro-Spheres as a Core Material for Dry Powder Layering of an API to Facilitate High API
API layering onto multi-particulate core materials has become an increasingly popular method of drug delivery in the pharmaceutical industry in recent years. The ability to control dosing, customize the dosing rate and reduce the risk of dose dumping along with the ability to create orally disintegrating tablets (ODT’s) with controlled release particles are all advantages to multi-particulate dosage forms.