The focus of this symposium was around Enabling Technologies and Innovation within the pharmaceutical industry. During this one-day event, there were presentations on a range of topics around formulation development, process development and production.
Freund-Vector’s Process Development Scientist, Prachi Shah led the discussion on “New Trends in Pharmaceutical Production: Continuous Granulation Processes.” Prachi’s presentation covered three main areas:
- Definition of Granulation and Continuous Manufacturing
- Equipment for Continuous Manufacturing
- Impacts of Formulation and Process Variables
Definition of Granulation and Continuous Manufacturing
Granulation is a process where loose powders are bound together to form a larger agglomerate. The status of granulation is as follows:
- Multiple unit operations
- Batch process
- Expensive, time consuming and labour intensive
- Large amounts of excipients needed to overcome issues with compactibility, flow, bulk density
- Extensive Scale Up trials
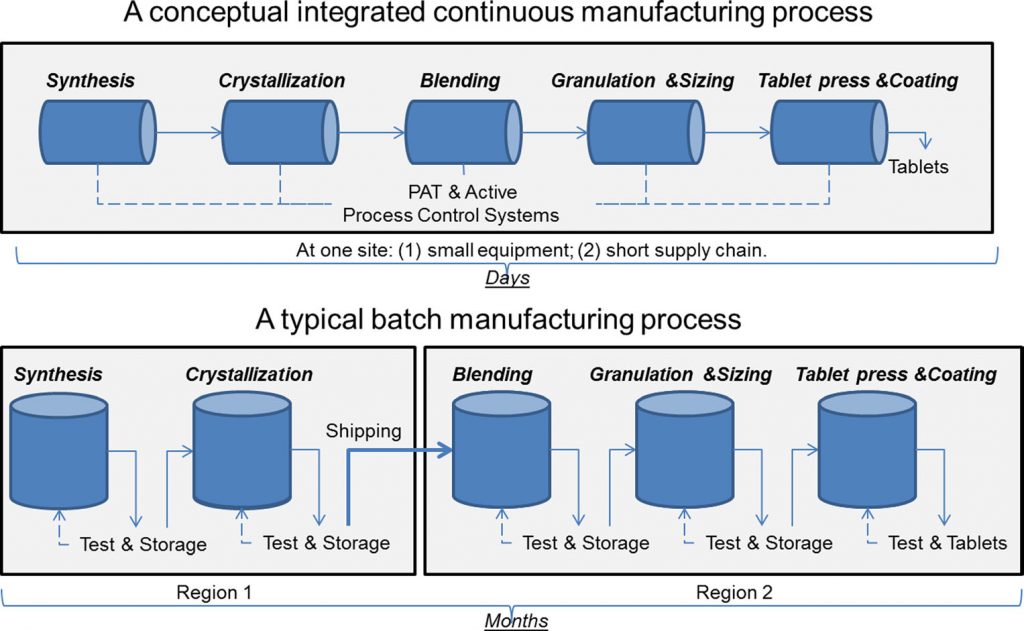
True continuous manufacturing (CM) is more than just a method of manufacturing in which materials are continuously being fed and removed from a process. It is the ultimate application of quality by design (QbD). The process is developed as an integrated whole with a systems approach, together with model-based design and control. The status of continuous manufacturing (CM) is best described by Michael Kopcha, Ph.D., R.Ph., FDA’s Director, Office of Pharmaceutical Quality, Center for Drug Evaluation and Research, “With many companies now evaluating their operations for potential uses of CM, some have found specific ways to utilize CM techniques in their own production processes. As a result of these individual efforts, we now see a variety of different approaches for implementing CM technology throughout industry. (Source: FDA Voice)
Equipment for Continuous Manufacturing
Freund-Vector has developed the Granuformer®, a continuous granulation and drying system. Continuous granulation occurs by a twin extruder and vertical granulator while the continuous drying is done by a spiral dryer. The Granuformer® produces granules equivalent of fluid bed granules, perfect for tableting. To learn more about Granuformer®, you can watch a video on Freund-Vector’s YouTube Channel.

Impacts of Formulation and Process Variables
Ashland and Freund-Vector conducted a pilot study “Continuous Granulation of Acetaminophen” using the Granuformer®. The goal of this pilot study was to evaluate important formulation and process conditions for continuous granulation.
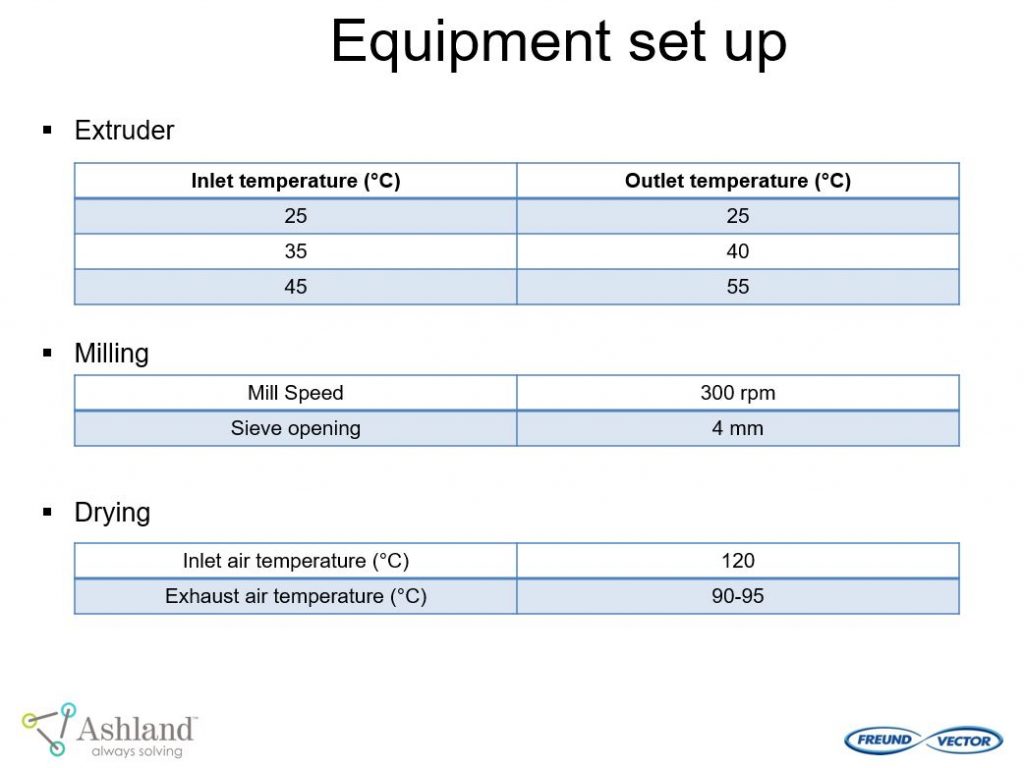
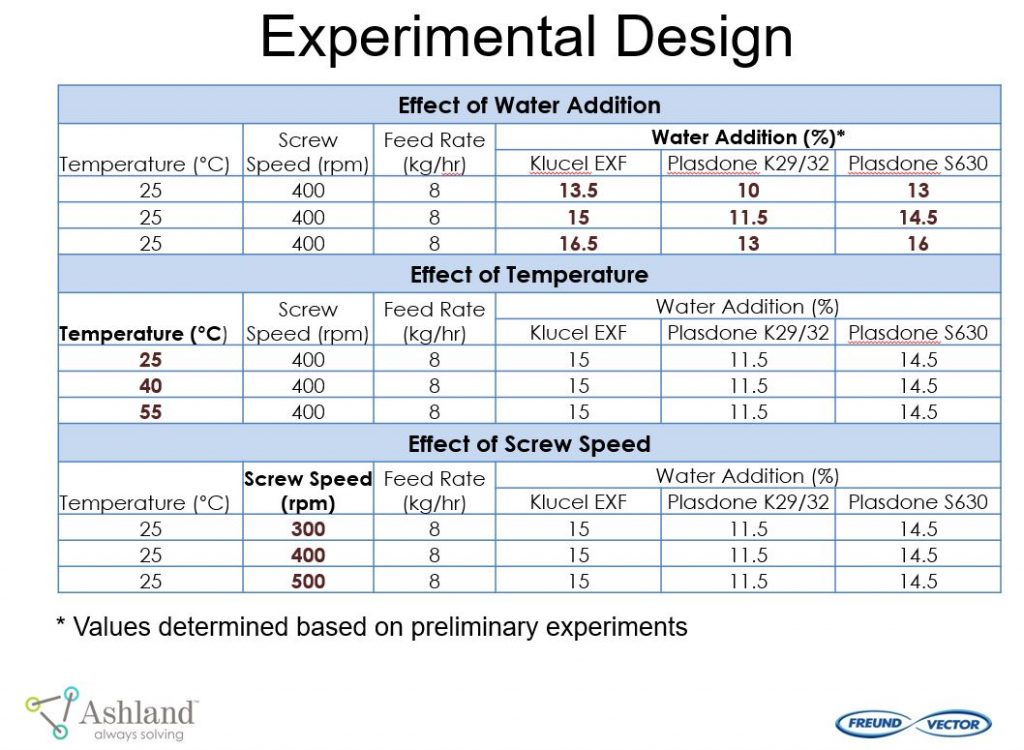
This pilot study provided the following conclusions:
- Continuous granulation is capable of making stronger, yet fast-dissolving tablets.
- All polymers yielded robust formulations and there was minimum effect of process variables on tablet hardness
- Granuformer® provides high mixing intensity, improved tablet strength and robustness.
