Control Systems For Pharmaceutical Processing Equipment
FREUND provides a full range of control systems for the operation of the equipment.
Our control systems are designed to meet the precise hardware and software requirements of the customer using disciplined and documented procedures. The control design system is based on proven process technology to control process parameter, system monitoring, alarming, feedback, and data acquisition. Validation documentation and field verification services are available for all control systems.
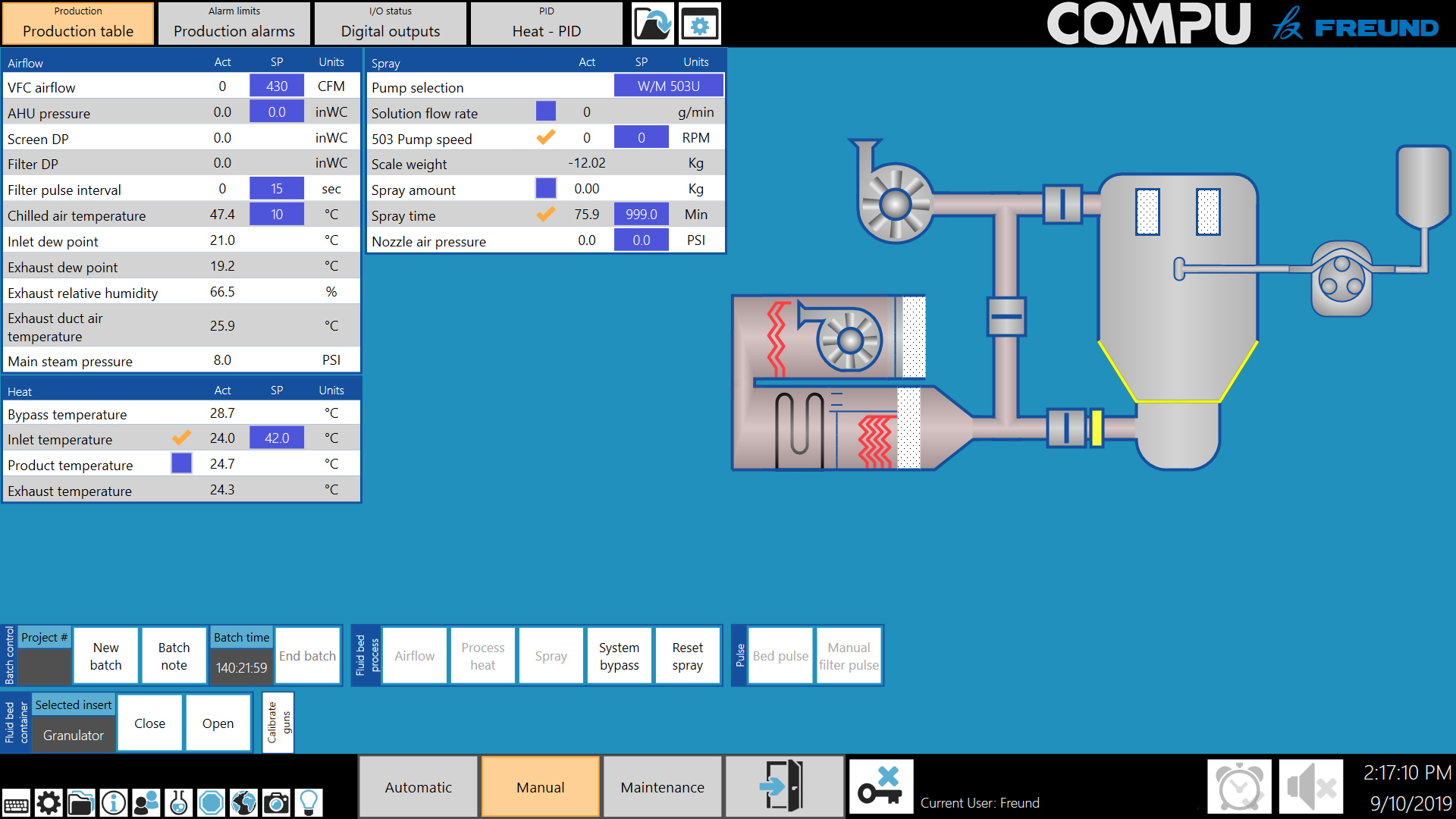
The COMPU™ automated process control system is designed specifically for pharmaceutical processing equipment.
Capable of displaying different languages at the setting of a switch, the COMPU™ is ideally suited throughout the world.
Designed to meet the strictest interpretation of 21 CFR Part 11, the COMPU™ can be configured to any pharmaceutical manufacturing and research facility.
The COMPU™ includes a patented Recipe Editor and incorporates Snapshot! Technology, which creates automatic recipes from historical batch reports.
With hundreds of validated systems installed, the COMPU™ provides the highest level of pharmaceutical processes control.
COMPU™ Features Include:
Operator-Friendly Interface
- Automatic (Recipe-Driven) and Manual Modes
- Supports up to 26 Different Languages
- Controls Multiple Processing Equipment:
- Ideal for Granulation Suites and Production Facilities
- Optional Touchscreen OIP Available
Patented Snapshot! Technology
- Import Manual Mode Set Points as a Recipe Step
- Import Any Batch Report as a Complete Recipe
Designed for 21 CFR Part 11
- Electronic Management for All Data and Records: Recipes, Batch Reports, Alarms, Security Files
Patented Recipe Management System
- Unlimited Number of Recipes and Recipe Templates
- Fully Configurable Transition Editor
GAMP Software Design Methods
- Full Validation “Dry Run” Prior to Shipment
Configurable Security System
- Individual Login Names and Passwords with Password Expiration Control
Open System Architecture
- PLC: Allen Bradley
- SCADA System: iFix, InTouch, or RSView Studio
- Download Recipes or Export Batch Reports to MES Systems or Remote Networks
Controls Retrofit and System Integration
As an industry process control technology leader, FREUND offers all the control packages as retrofits for competitive processing systems along with updates to existing Freund systems.
Validation Services
FREUND provides validation services for the support of processing equipment and control systems.
Validation services for FREUND equipment lines include comprehensive testing protocol for Installation Qualification (IQ) and Operational Qualification (OQ). FREUND can also provide Process Qualification (PQ) assistance when situations dictate the need for additional validation services.
FREUND maintains complete confidentiality of any proprietary processes involved in the production of pharmaceutical goods. We believe that to have a “validated” process, one must start out with software and hardware that is under design scrutiny and control prior to validating the system. Validated systems are not tested, they are designed and built from the very beginning under controlled conditions.